|
|
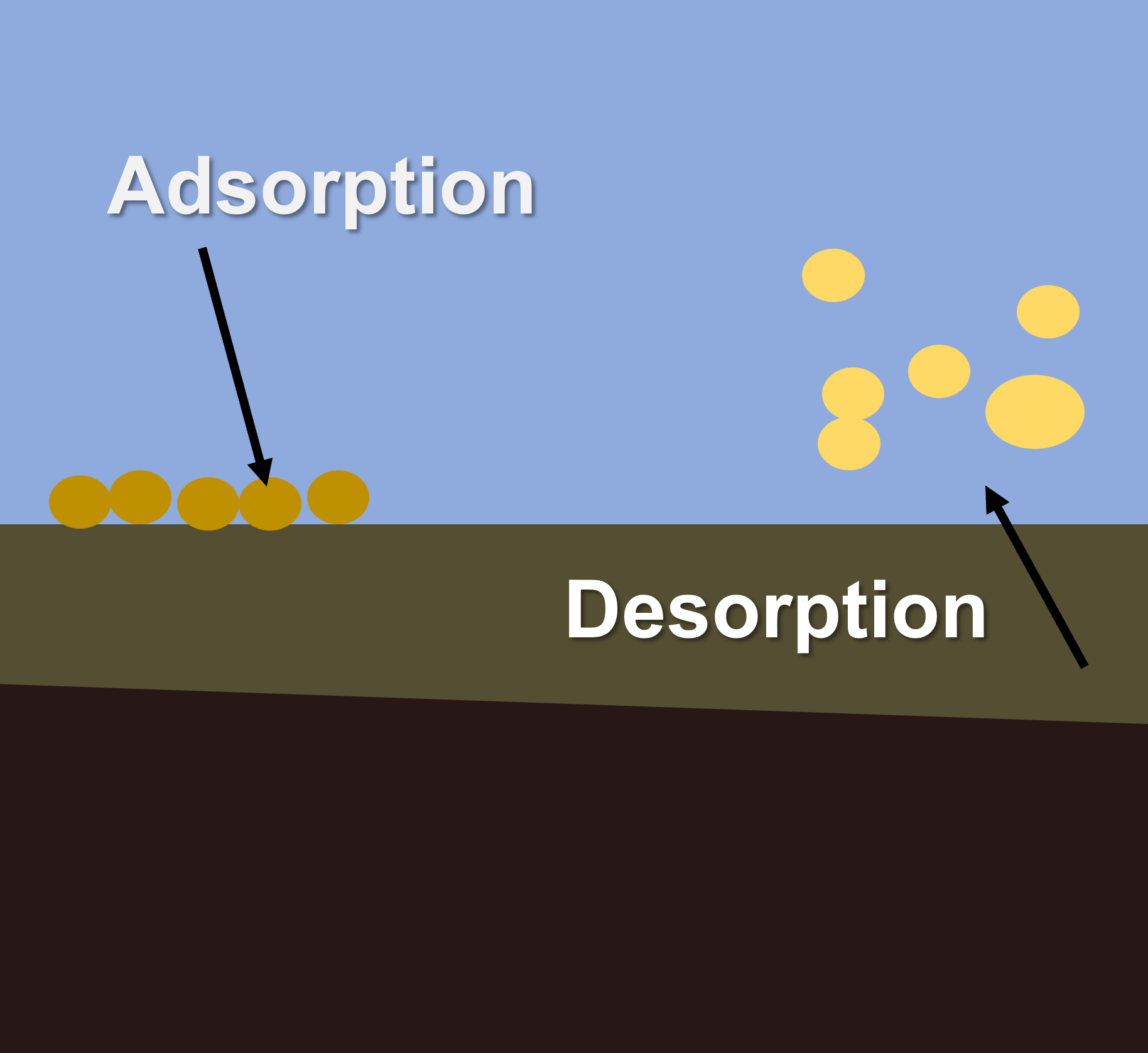
Adsorption and DesorptionAdsorption and desorption can occur on the surface of soil and sediment particles, and organic matter. Nutrients, metals, salts and pesticides may adsorb to sediment particles and organic matter, impacting processes of bioavailability, transport and toxicity. Adsorption and desorption processes play important roles in nutrient availability in soils and water. In soils, clay minerals and organic matter have high surface areas and generally possess negative surface charge. This provides sites for positively charged ions (cations) to be electrostatically attracted to the surface and adsorb. Positive ions include nutrients such as ammonia (NH4+), potassium (K+), calcium (Ca2+) and magnesium (Mg2+). Desorption of these cations can occur when they are replaced by other cations. The capacity of a material (such as a soil) to hold exchangeable cations is termed its ‘cation exchange capacity’ and is an important indicator of soil fertility and the supply of nutrient to plants and other biota[5][3]. Ammonium can be adsorbed to fine particles in soils, which can then be are eroded during rainfall events and be transported through river systems. In this adsorbed form they are relatively unavailable for biotic uptake. However, through ion exchange with saline water, this adsorbed ammonium can be released (desorbed) into estuarine or coastal waters where it can drive primary productivity and algal growth[4]. Adsorption of heavy metals and pesticides can impact their toxicity in the environment by inhibiting their movement through soils and sediment, and making them less bioavailable. Adsorption of metals to suspended solids can also enhance their removal from waters as sedimentation of particulates may remove them from the water column and store them in sediments. References
Last updated: 16 October 2023 This page should be cited as: Department of Environment, Science and Innovation, Queensland (2023) Adsorption and Desorption, WetlandInfo website, accessed 18 March 2024. Available at: https://wetlandinfo.des.qld.gov.au/wetlands/ecology/processes-systems/adsorption/ |

 — Department of Environment, Science and Innovation
— Department of Environment, Science and Innovation